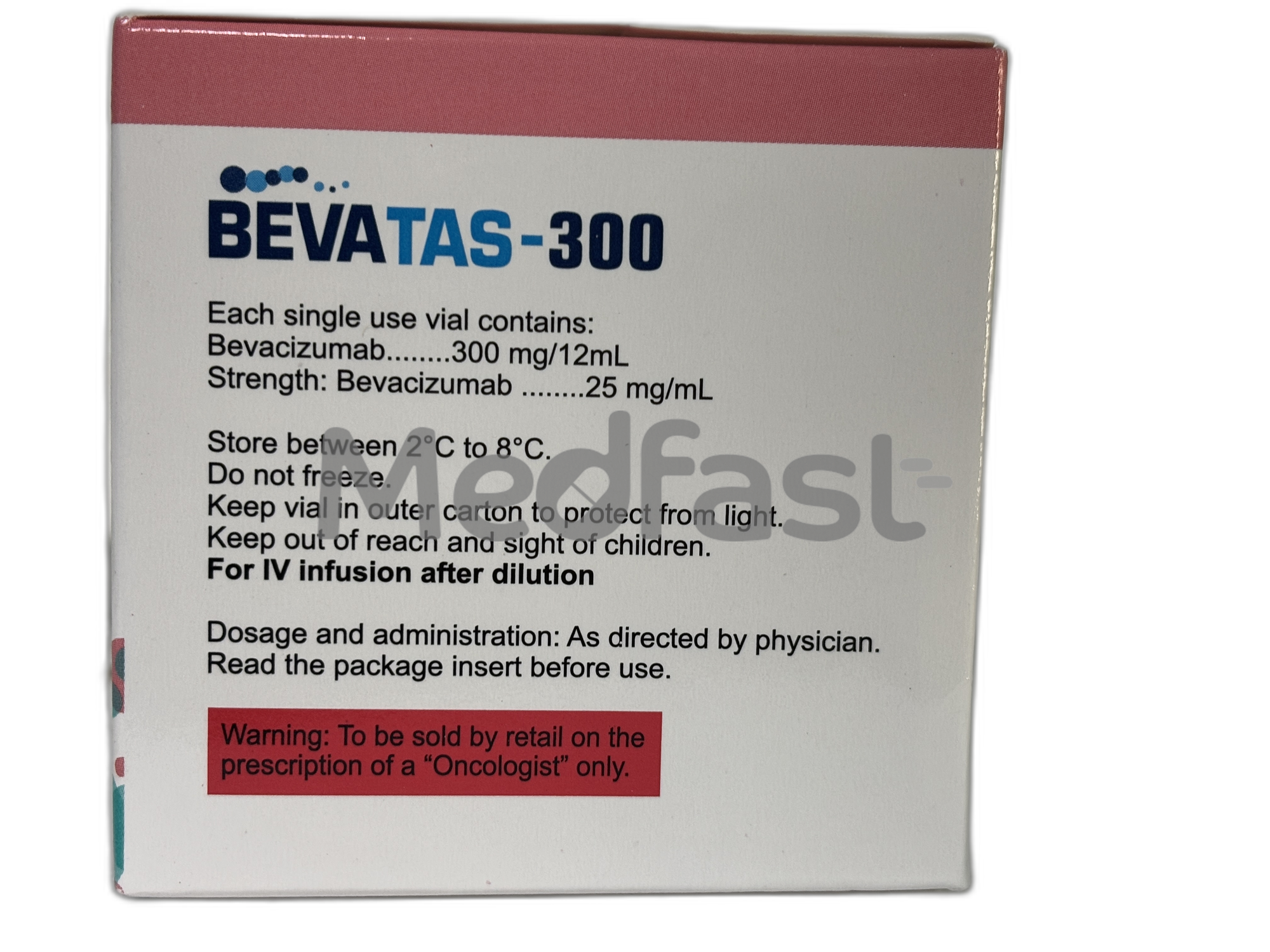
Bevatas 300mg Injection contains Bevacizumab as its active component and is classified as an anticancer medication. It works by inhibiting the formation of blood vessels that supply nutrients to tumors, thereby slowing or stopping their growth. This injection is used in combination with other chemotherapy drugs to manage a variety of advanced or metastatic cancers, including breast cancer, brain tumors (such as glioblastoma), cervical cancer, kidney (renal cell) cancer, lung cancer, and colorectal cancer. It is also indicated for cancers involving the ovaries, fallopian tubes, or the peritoneum.
Women of childbearing age should use reliable contraception during treatment and for at least six months following the final dose, as the drug can harm an unborn baby and potentially affect fertility. Patients who are planning to conceive in the future should consult their physician regarding the possibility of fertility preservation. Additionally, breastfeeding is not recommended during treatment due to the risk of interference with the infant’s growth and development.
Bevatas 300mg Injection is typically administered under medical supervision in a hospital or clinical setting. It is often given alongside other chemotherapy agents, and common side effects include constipation, decreased appetite, nasal congestion, fever, various skin issues, and weight loss. Prior to initiating treatment, it is essential to inform your healthcare provider if you have a history of hypertension, elevated blood sugar levels, gastrointestinal conditions, or cardiovascular problems.
Because Bevacizumab can impair wound healing and increase the risk of bleeding, it should not be administered for at least 28 days before or after any surgical procedure. It is important to inform your doctor if you require any dental surgeries as well. If you experience symptoms such as blurred vision, confusion, or other neurological changes during the course of treatment, seek immediate medical advice to avoid serious complications