- Multiple myeloma (cancer of a kind of white blood cell called plasma cell)
- Hodgkin’s disease (lymphoid tumor)
- Non-Hodgkin’s lymphomas (lymphoid tumors)
- Brain tumors (medulloblastoma, glioblastoma, astrocytoma, and metastatic brain tumors)
CARSTIM
| MRP | : |
|
| Price | : | ₹4,600.00 |
| You Save | : | ₹1,988.00 (30.18%) |
1 Vial(s)
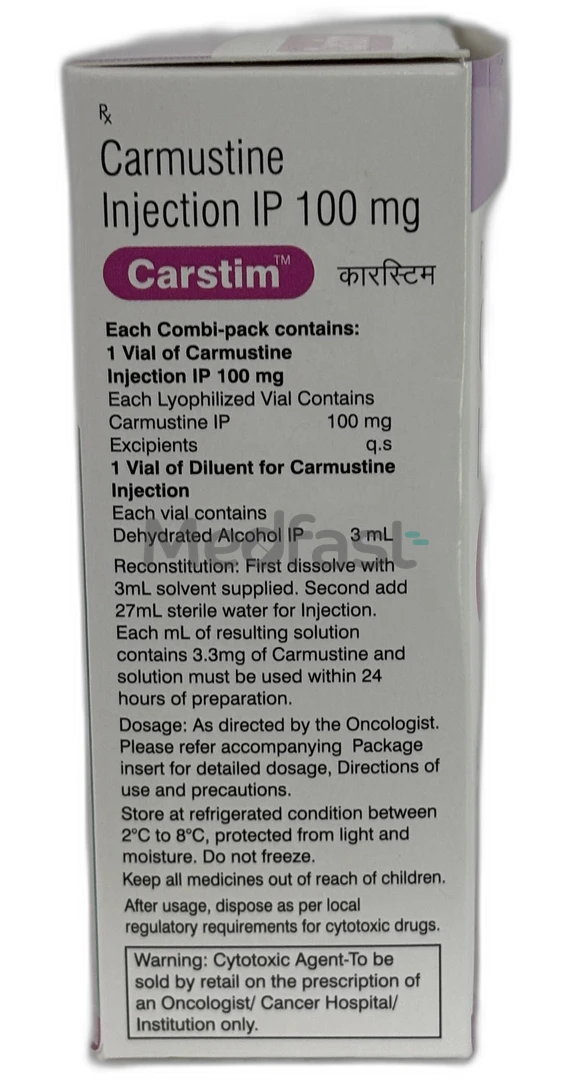
Carstim 100 mg Combipack Injection 1’s is a chemotherapeutic agent utilized for treating brain tumors. A tumor is characterized as a solid mass of tissue resulting from the abnormal proliferation of cells. A brain tumor represents a serious medical condition where cells, whether cancerous or non-cancerous, grow irregularly within the brain. Additionally, Carstim 100 mg Combipack Injection 1’s is employed in the treatment of multiple myeloma alongside prednisone, as well as Hodgkin’s Disease and non-Hodgkin’s Disease in conjunction with other approved medications.
Carstim 100 mg Combipack Injection 1’s contains Carmustine, which is classified as an alkylating agent. Its mechanism of action involves the inhibition of DNA and RNA transcription, which is crucial for protein synthesis. This action leads to the reduction of tumor cells, thereby hindering the growth and further dissemination of both cancerous and non-cancerous cells.
Certain side effects may occur with Carstim 100 mg Combipack Injection 1’s, including nausea, vomiting, headaches, pale skin, dizziness, stomach upset, fatigue, loss of balance, and pain at the injection site. These side effects typically do not necessitate medical intervention and tend to resolve on their own over time. However, if these effects persist or intensify, it is advisable to consult your physician. Carstim 100 mg Combipack Injection 1’s is administered parenterally by a qualified healthcare professional, and self-administration is not recommended.
Individuals who are allergic to Carstim 100 mg Combipack Injection 1’s or any of its components should avoid its use. It is important to inform your doctor of your complete medical history, including any current medications, to prevent potential side effects or interactions. Prior to administration, notify your doctor if you have liver or kidney disease, heart conditions, or respiratory issues. In some patients, Carstim 100 mg Combipack Injection 1’s may lead to pulmonary toxicity, bone marrow suppression, gastrointestinal toxicity, nephrotoxicity, and hepatotoxicity. Therefore, careful monitoring is essential throughout the treatment process. This medication is also known to pose risks of fetal harm during pregnancy.